Table of Contents
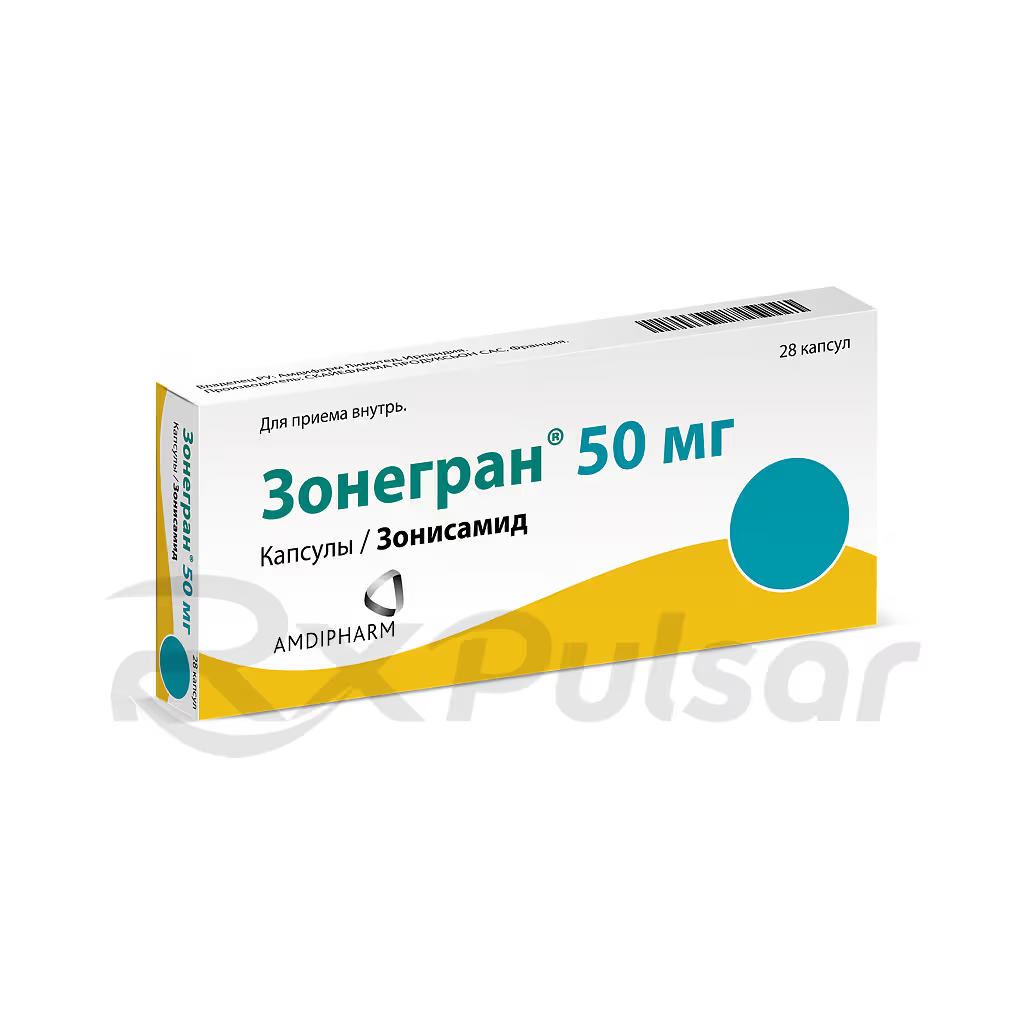
ZONEGRAN™ 50mg 28 Capsules Buy Online
Zonegran (Zonisamide) Capsules: A Comprehensive Overview
Zonegran, containing the active ingredient zonisamide, is an anticonvulsant medication used to manage certain types of seizures. Its effectiveness lies in its ability to modulate neuronal excitability, helping to control the abnormal electrical activity in the brain that triggers seizures.
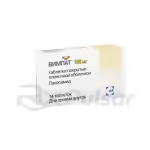
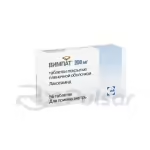
This medication is available in capsule form, offering a convenient method of administration. The 50mg capsules, packaged in quantities of 28, provide a precise and consistent dosage. The recommended dosage and administration will be determined by a healthcare professional based on individual patient needs.
Zonegran is frequently used as an adjunctive therapy, meaning it’s combined with other anti-seizure medications to enhance their effectiveness. It’s also used in monotherapy for certain adult patients with partial seizures.
Understanding the mechanism of action, pharmacokinetics, potential benefits, and drawbacks associated with Zonegran is crucial for informed treatment decisions. This overview will shed light on these key aspects to aid in comprehensive patient care.
Understanding Zonegran
Zonegran, containing the active ingredient zonisamide, is a crucial anticonvulsant medication used in the management of epilepsy. It’s classified as a sulfonamide derivative, distinct in its mechanism of action from many other anti-seizure drugs. This unique approach makes it a valuable tool in managing various seizure types, particularly when other treatments have proven insufficient.
The precise mechanism by which zonisamide exerts its anticonvulsant effects is not fully elucidated, but it’s believed to involve multiple pathways. Studies suggest that it may affect the sodium channels in neurons, reducing their excitability. Additionally, it’s thought to modulate the release of neurotransmitters, influencing the overall balance of brain activity and potentially preventing the spread of seizure activity.
Importantly, Zonegran is often prescribed as an adjunctive therapy, supplementing other anti-epileptic drugs already in use. This combination approach can often prove more effective than monotherapy, particularly in patients with complex or poorly controlled seizures. However, monotherapy with Zonegran may be considered for specific adult patient populations with partial-onset seizures.
Its role in epilepsy management is significant, offering a potential solution for individuals who haven’t found adequate seizure control with other medications. Therefore, understanding Zonegran’s unique properties and clinical applications is paramount for healthcare professionals treating epilepsy.
Dosage and Administration
Zonegran capsules are administered orally, swallowed whole with water. Dosage is highly individualized and should always be determined by a healthcare professional, carefully considering the patient’s specific needs and response to treatment. It’s crucial to follow the prescribed dosage regimen precisely, as adjustments may be necessary to optimize therapeutic benefits while minimizing potential adverse effects.
Typically, treatment begins with a low initial dose, gradually increasing over time until the optimal therapeutic level is reached. This titration process allows for careful monitoring of the patient’s response and minimizes the risk of adverse reactions. The maximum recommended daily dose is 500mg, though this may not be appropriate for all patients.
The frequency of administration can vary depending on the individual’s needs and the physician’s judgment. Some patients may require divided doses throughout the day, while others may tolerate a once-daily regimen. Consistent adherence to the prescribed schedule is essential for maintaining therapeutic blood levels and achieving optimal seizure control.
Close monitoring by a healthcare provider is vital throughout the treatment process. Regular follow-up appointments allow for assessment of the drug’s efficacy, adjustment of the dosage as needed, and management of any potential side effects. Patients should promptly report any new or worsening symptoms to their physician.
Therapeutic Use
Zonegran’s primary therapeutic application lies in the management of epilepsy. Specifically, it’s indicated for the treatment of partial-onset seizures, both with and without secondary generalization. This means it can be effective in controlling seizures that originate in a specific area of the brain and may or may not spread to involve the entire brain.
In many cases, Zonegran is employed as an adjunctive therapy, meaning it’s used in conjunction with other anti-epileptic drugs. This combination approach can often lead to better seizure control than using a single medication alone, particularly in patients with complex or treatment-resistant epilepsy.
While primarily used in adults, Zonegran may also be considered for children aged six years and older with partial-onset seizures, usually as adjunctive therapy. The decision to use Zonegran in pediatric patients should be made on a case-by-case basis by a qualified healthcare professional, carefully weighing the potential benefits against the risks.
The efficacy of Zonegran in achieving seizure freedom varies among individuals. Careful monitoring of seizure frequency and severity is essential to assess the drug’s effectiveness. Dosage adjustments may be necessary to optimize therapeutic benefit, and alternative treatments may need to be considered if Zonegran proves insufficient in controlling seizures.
Mechanism of Action
While the precise mechanism of Zonegran’s anticonvulsant action isn’t fully understood, research suggests it involves multiple complex interactions within the central nervous system. It’s believed to modulate the activity of voltage-gated sodium channels in neurons, thereby reducing their excitability and preventing the excessive firing that characterizes seizures.
Furthermore, studies indicate that Zonegran may also influence the release of various neurotransmitters, impacting the delicate balance of neuronal activity. This modulation of neurotransmission may contribute to its effectiveness in preventing or suppressing seizure propagation. The exact neurotransmitter systems affected and the precise nature of these interactions remain areas of ongoing research.
Its effects on carbonic anhydrase, an enzyme involved in regulating pH balance in the brain, have also been investigated. Although Zonegran’s inhibition of carbonic anhydrase is relatively weak compared to other drugs in this class, this interaction could contribute to its overall anticonvulsant effects. The interplay of these different mechanisms likely underlies its broad-spectrum activity.
The multifaceted nature of Zonegran’s mechanism of action contributes to its effectiveness in managing diverse seizure types. Further research continues to unravel the complexities of its interactions with neuronal pathways, providing a more complete understanding of its therapeutic potential in epilepsy.
Pharmacokinetics
Following oral administration, zonisamide, the active component of Zonegran, is readily absorbed from the gastrointestinal tract. Peak plasma concentrations are typically achieved within 2 to 4 hours, although this can be influenced by factors such as food intake and individual patient variations. The extent of absorption is generally high, ensuring consistent drug delivery to the target sites.
Zonisamide exhibits a high degree of protein binding in the plasma, with over 95% bound to proteins. This characteristic has implications for drug interactions, as it can affect the free concentration of zonisamide available to exert its therapeutic effects. It’s also important to note that this extensive protein binding can lead to drug-drug interactions with other highly protein-bound medications.
The drug undergoes significant metabolism in the liver, primarily through glucuronidation and oxidation pathways. This metabolic process results in the formation of various metabolites, some of which may possess minor pharmacological activity. The primary route of excretion is through the urine, with a substantial portion of the drug and its metabolites eliminated within 24 hours.
The elimination half-life of zonisamide is relatively short, typically ranging from 5 to 7 hours. This relatively rapid elimination means that steady-state plasma concentrations are usually reached within a few days of initiating therapy at a stable dose. This pharmacokinetic profile influences dosage regimens and the frequency of administration.
Pros of Zonegran
Advantages
Zonegran offers several advantages in the management of epilepsy. Its unique mechanism of action, distinct from many other anticonvulsants, makes it a valuable option for patients whose seizures are not adequately controlled by other medications. This difference in mechanism can lead to improved seizure control when used alone or in combination with other therapies. The relatively short half-life allows for rapid adjustments to dosage, enabling quicker optimization of therapy.
Many patients experience a significant reduction in seizure frequency following the initiation of Zonegran therapy. This improved seizure control can lead to a substantial enhancement in quality of life, allowing individuals to participate more fully in daily activities and experience fewer disruptions from their condition. For some individuals, Zonegran may even lead to complete seizure freedom.
Furthermore, Zonegran’s generally well-tolerated profile, at least in comparison to some other anti-seizure medications, is a significant advantage. While side effects can occur, they are often manageable and not severe enough to necessitate treatment discontinuation for many patients. This makes it a suitable option for long-term management in many cases.
Finally, the availability of Zonegran in various dosage forms, including capsules, can enhance patient compliance and convenience. This ease of administration can be particularly important for individuals who may struggle with swallowing larger pills or those who need a flexible dosing schedule. These factors can contribute to better long-term seizure management.
Advantages
One key advantage of Zonegran is its potential to significantly reduce seizure frequency in patients with partial-onset seizures. This improved seizure control can translate to a marked improvement in a patient’s quality of life, allowing for greater participation in daily activities and reduced disruption caused by seizures. For some, it may even lead to complete seizure freedom, a life-changing outcome.
The relatively short elimination half-life of Zonegran offers a significant benefit. This allows for quicker adjustments in dosage if needed, facilitating a more rapid optimization of therapy. This characteristic is especially valuable when initiating treatment or making adjustments to address side effects or lack of efficacy.
Another advantage stems from Zonegran’s unique mechanism of action, differing from many other anti-epileptic drugs. This distinction can be particularly beneficial for individuals whose seizures are not effectively controlled by other medications. Its multi-faceted approach allows for a broader impact on various aspects of seizure activity.
Finally, the generally well-tolerated nature of Zonegran, compared to some other anticonvulsants, is a notable advantage. While side effects are possible, they are often manageable, leading to better patient compliance and adherence to the prescribed treatment regimen, crucial for long-term seizure control. This improved tolerability contributes to successful long-term epilepsy management.
Cons of Zonegran
Disadvantages
While Zonegran offers significant benefits for many, potential drawbacks should be considered. One common concern is the occurrence of neurological side effects, such as dizziness, drowsiness, and headache. These effects are often dose-related, meaning they may be more pronounced at higher doses. Careful titration of the dosage can help to minimize these adverse events.
Another potential side effect is kidney stones. This risk is increased in patients with a history of kidney stones or those who are dehydrated. Maintaining adequate hydration and monitoring renal function are important preventive measures. The risk of kidney stones necessitates careful consideration and monitoring of renal function.
Furthermore, Zonegran can interact with other medications, particularly those that are highly protein-bound. This interaction can alter the plasma concentrations of either drug, potentially leading to either reduced efficacy or increased risk of side effects. Healthcare professionals should carefully review a patient’s medication list to identify potential drug interactions.
Finally, while generally well-tolerated, some patients may experience gastrointestinal disturbances, such as nausea and vomiting. These side effects can sometimes be mitigated by taking the medication with food. However, if these symptoms prove persistent or severe, adjustment of the dosage or alternative treatment may be necessary. It is crucial to report any significant side effects to your physician.
Disadvantages
A significant potential drawback associated with Zonegran is the risk of developing kidney stones. This risk is heightened in individuals with a pre-existing history of kidney stones or those who are prone to dehydration. Maintaining adequate hydration is crucial to mitigate this risk, and careful monitoring of renal function is recommended during treatment.
Several neurological side effects are commonly reported, including dizziness, drowsiness, and headache. The severity of these side effects often correlates with the dosage; therefore, a gradual increase in dosage, under medical supervision, is generally recommended to minimize the occurrence or severity of such symptoms. Careful monitoring and adjustment of the dosage is essential.
Zonegran’s extensive protein binding can lead to clinically significant drug interactions. This necessitates a thorough review of a patient’s medication list to identify potential incompatibilities. Concurrent use of other highly protein-bound medications may necessitate dosage adjustments or alternative treatment strategies to prevent adverse effects.
Finally, gastrointestinal issues such as nausea and vomiting have been reported in some patients. While these side effects are often mild and transient, they can still significantly impact a patient’s quality of life. Strategies to alleviate these symptoms, such as taking the medication with food, may be considered; however, if symptoms persist, medical consultation is recommended to explore alternative management options.
Additional Considerations
Composition and Packaging
Zonegran capsules contain the active pharmaceutical ingredient zonisamide. Each capsule provides a precise dose, ensuring consistent medication delivery. The specific dosage strength will vary depending on the prescription, but commonly available strengths include 25mg, 50mg, and 100mg capsules. These variations allow for precise titration of the dosage to meet individual patient needs.
In addition to the active ingredient, Zonegran capsules contain various inactive excipients. These inactive components play a crucial role in maintaining the integrity and stability of the formulation. They may include fillers, binders, and other substances necessary for the manufacturing process. The precise composition of these excipients can be found in the detailed product information provided by the manufacturer.
Zonegran 50mg capsules are typically packaged in blister packs to protect the medication from environmental factors and ensure its stability. These blister packs are often further contained within a sturdy cardboard carton for added protection during shipping and storage. The packaging clearly displays essential information such as the drug name, dosage, quantity, and manufacturer’s details.
Careful handling and storage of Zonegran capsules are important to maintain their quality and efficacy. Storing the medication in a cool, dry place, away from direct sunlight and moisture, is recommended. Always follow the storage instructions provided on the product label to ensure the long-term stability of the medication and its continued effectiveness.
-
 Georgia Austin [Author]
Georgia Austin [Author]Georgia Austin is a seasoned SEO content writer, editor, and content marketing strategist with over 7 years of experience crafting compelling copy for leading brands in the healthcare and pharmaceutic...
View all posts
-
 Jonathan Brown [Editor]
Jonathan Brown [Editor]Jonathan Brown is a seasoned professional editor, researcher, and educator with over 12 years of experience helping authors find their voice and polish their writing. As a content editor for RxPulsar....
View all posts
-
 Elizabeth Dennis, MD [Medical reviewer]
Elizabeth Dennis, MD [Medical reviewer]Dr. Elizabeth Dennis is a highly skilled Orthopedic Surgeon and consultant for RxPulsar.com, a licensed online pharmacy. She specializes in the management and surgical treatment of knee, shoulder, and...
View all posts





















Reviews
There are no reviews yet.