Table of Contents
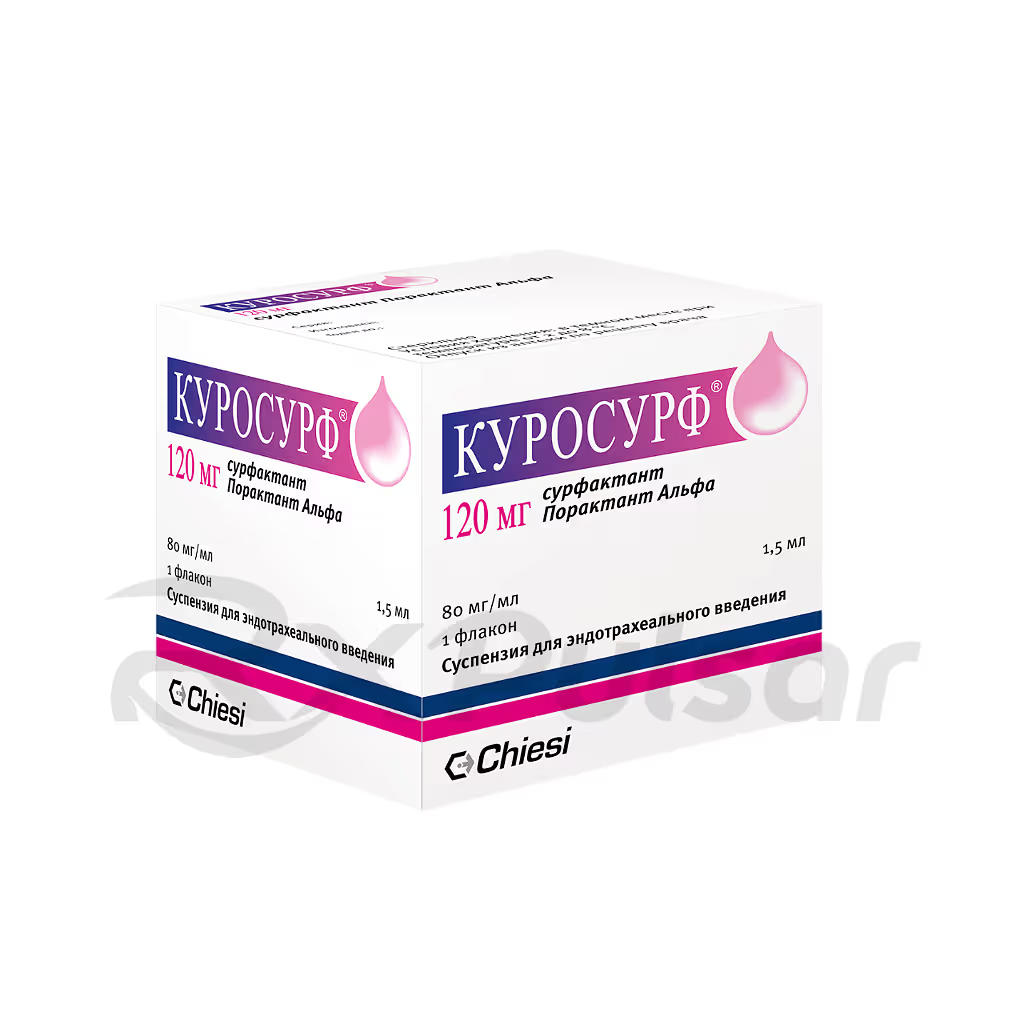
CUROSURF™ 80mg Suspension 1.5ml Buy Online
CUROSURF: A Lifesaving Pulmonary Surfactant
Respiratory Distress Syndrome (RDS) in premature infants is a critical condition requiring immediate intervention. CUROSURF, a poractant alfa-based pulmonary surfactant, offers a vital lifeline in these situations, helping to restore normal lung function and improve survival rates.
This naturally derived surfactant, extracted from porcine lungs, mimics the body’s own substance, reducing surface tension in the alveoli. This crucial action facilitates easier breathing and prevents alveolar collapse, a hallmark of RDS.
CUROSURF is administered directly into the lungs via endotracheal instillation. The precise dosage and frequency depend on the infant’s weight and clinical response, as determined by a healthcare professional. Careful monitoring is essential throughout the treatment process.
The efficacy of CUROSURF has been demonstrated in numerous clinical studies. It’s a cornerstone treatment for RDS in premature infants, often improving oxygenation and reducing the need for mechanical ventilation.
While generally safe and effective, potential risks associated with CUROSURF administration exist. These include, but are not limited to, transient bradycardia and hypotension. Therefore, close monitoring of vital signs is crucial during and after administration.
Understanding CUROSURF
CUROSURF is a pulmonary surfactant replacement therapy indicated for the treatment of Respiratory Distress Syndrome (RDS) in premature infants. This life-saving medication addresses the critical deficiency of surfactant in the lungs of these vulnerable newborns. The absence of this crucial substance leads to alveolar collapse and severe breathing difficulties, a condition often life-threatening.
The active ingredient in CUROSURF is poractant alfa, a complex mixture of phospholipids and specific proteins. This formulation closely mirrors the natural surfactant found in healthy lungs. It’s derived from porcine lungs through a rigorous purification process, ensuring both efficacy and safety.
Unlike synthetic surfactants, CUROSURF’s natural composition ensures a more physiological interaction with the infant’s lungs. This natural approach is designed to minimize adverse reactions and optimize therapeutic effectiveness. The product comes in ready-to-use vials, eliminating the need for reconstitution and simplifying administration.
CUROSURF is specifically formulated as an endotracheal suspension, meaning it’s delivered directly into the infant’s lungs through an endotracheal tube. This method of administration ensures the surfactant reaches the alveoli directly, where it is most needed to restore proper lung function. The precise dosage is determined by the infant’s weight and clinical condition, as directed by a healthcare professional.
The unique properties of CUROSURF make it a valuable tool in the neonatal intensive care unit. Its natural composition and ease of administration contribute to its widespread use and clinical success in managing RDS. However, as with any medication, potential side effects warrant careful monitoring of the infant’s vital signs during and after administration.
Mechanism of Action
CUROSURF’s therapeutic effect stems from its ability to restore the crucial function of pulmonary surfactant in the lungs of premature infants. Pulmonary surfactant is a complex mixture of lipids and proteins naturally produced by the lungs. It’s essential for reducing surface tension within the alveoli, the tiny air sacs where gas exchange occurs.
In premature infants with Respiratory Distress Syndrome (RDS), the production of surfactant is insufficient or absent, leading to alveolar collapse and significant respiratory distress. CUROSURF, containing poractant alfa, directly addresses this deficiency. The administered poractant alfa integrates into the alveolar lining, effectively reducing surface tension.
By lowering surface tension, CUROSURF prevents alveolar collapse and improves lung compliance. This means the lungs can expand and contract more easily, facilitating efficient gas exchange. The improved gas exchange leads to increased oxygen uptake and reduced carbon dioxide retention, alleviating the respiratory distress experienced by the infant.
The specific components of poractant alfa, namely the phospholipids and surfactant-associated proteins (SP-B and SP-C), play distinct roles in this process. Phospholipids primarily contribute to the reduction in surface tension, while SP-B and SP-C are essential for the proper adsorption and spreading of the surfactant film on the alveolar surface. This intricate interplay ensures the effective restoration of normal lung function.
The overall effect is a significant improvement in respiratory mechanics, oxygenation, and overall clinical condition. This mechanism of action makes CUROSURF an invaluable treatment option for premature infants suffering from RDS, offering a direct and effective way to address the underlying cause of their respiratory distress. The rapid onset of action contributes to its clinical efficacy.
Dosage and Administration
CUROSURF administration requires meticulous attention to detail and should only be performed by trained healthcare professionals experienced in neonatal resuscitation and surfactant administration. The medication is intended for endotracheal instillation, meaning it’s delivered directly into the trachea via an endotracheal tube.
Dosage is determined based on the infant’s weight and clinical condition. The recommended starting dose is typically in the range of 100-200 mg/kg, administered as a single dose or in divided doses, depending on the infant’s response. A healthcare professional will carefully calculate the appropriate dose based on the individual needs of the infant.
Prior to administration, the CUROSURF suspension should be gently agitated to ensure uniform distribution of the poractant alfa. The vial is for single use only; any unused portion must be discarded. The suspension should be administered slowly and evenly into the trachea, avoiding rapid instillation which could lead to complications.
Following administration, careful monitoring of the infant’s respiratory status, including oxygen saturation, heart rate, and blood pressure, is crucial. Observation for any adverse reactions, such as bradycardia or hypotension, is essential. Prompt intervention may be necessary in case of complications. The treatment is a collaborative effort between neonatologists, respiratory therapists, and nurses.
The specific administration techniques, including the method of instillation and the use of specialized equipment, are guided by established clinical protocols. These protocols are designed to optimize the delivery of CUROSURF and minimize the potential for adverse events. Adherence to these guidelines is paramount to ensure patient safety and optimal treatment outcomes. Thorough documentation of the dosage, administration, and subsequent response is crucial.
Clinical Applications
CUROSURF’s primary clinical application lies in the treatment of neonatal respiratory distress syndrome (RDS), a critical condition affecting premature infants. RDS occurs due to a deficiency in pulmonary surfactant, leading to alveolar collapse and severe respiratory distress. CUROSURF’s ability to directly replace this deficient surfactant makes it a cornerstone treatment for this life-threatening condition.
The use of CUROSURF is particularly crucial in premature infants, as their lungs often lack the maturity necessary for adequate surfactant production. Early intervention with CUROSURF can significantly improve respiratory function, reducing the need for mechanical ventilation and improving overall survival rates. This timely intervention is vital for minimizing potential long-term complications associated with prolonged respiratory support.
Clinical studies have consistently demonstrated CUROSURF’s efficacy in improving oxygenation, reducing respiratory distress, and decreasing mortality rates in infants with RDS. Its effectiveness has led to its widespread adoption in neonatal intensive care units worldwide. The positive impact on clinical outcomes has made CUROSURF an integral part of modern neonatal respiratory care.
While primarily used for RDS, CUROSURF may also be considered in other clinical scenarios where surfactant deficiency is suspected. These situations might include meconium aspiration syndrome or other conditions affecting surfactant production or function in the neonate. However, the specific application and dosage in these scenarios should be determined on a case-by-case basis, guided by clinical expertise and established treatment guidelines.
The clinical utility of CUROSURF extends beyond immediate respiratory support. By improving lung function and minimizing respiratory distress, it can contribute to improved overall health and development in surviving infants. This positive impact on long-term outcomes underscores the importance of this therapy in neonatal intensive care.
Storage and Handling
Proper storage and handling of CUROSURF are critical to maintaining its efficacy and ensuring patient safety. The medication should be stored under refrigerated conditions, between 2°C and 8°C (36°F and 46°F). Exposure to higher temperatures can degrade the active components, compromising the product’s effectiveness. It’s crucial to avoid freezing.
Protection from light is equally vital. Exposure to light can also negatively impact the stability of the poractant alfa, reducing its potency. Therefore, the vials should be stored in their original packaging, shielded from direct sunlight or other sources of light. The storage area should be clean, dry and free from contamination.
Before administration, the vial should be inspected for any signs of damage, discoloration, or particulate matter. If any abnormalities are observed, the vial should be discarded and a new one used. The medication is intended for single-use only; any unused portion must be discarded after use, even if some of the contents remain in the vial. The use of sterile techniques is vital to prevent infection.
Once removed from refrigeration, the vial should be allowed to reach room temperature before use. Gently swirling the vial to ensure even distribution of the suspension is recommended, but vigorous shaking should be avoided. After administration, appropriate disposal procedures must be followed, in accordance with local regulations and guidelines for the disposal of pharmaceutical waste.
Healthcare professionals should strictly adhere to the manufacturer’s instructions for storage and handling. This will ensure the integrity and safety of the product, helping to maintain its therapeutic effectiveness and minimize the risk of adverse events. Comprehensive staff training on proper handling and storage procedures is vital for optimal results and patient safety.
Pros
Advantages of CUROSURF
CUROSURF offers several significant advantages in the treatment of neonatal respiratory distress syndrome (RDS). Its natural composition, closely mimicking endogenous pulmonary surfactant, ensures a physiological interaction with the infant’s lungs, potentially minimizing adverse reactions compared to synthetic alternatives. This natural approach is a key benefit.
The medication’s ready-to-use formulation simplifies administration, reducing preparation time and minimizing the risk of errors. This ease of use is particularly valuable in emergency situations requiring rapid intervention. The streamlined process contributes to efficient workflow in busy neonatal intensive care units.
Numerous clinical studies have demonstrated CUROSURF’s effectiveness in improving oxygenation, reducing the need for mechanical ventilation, and improving overall survival rates in infants with RDS. These positive clinical outcomes have established CUROSURF as a highly effective treatment option. The improved survival rates are a significant clinical advantage.
CUROSURF’s efficacy in reducing mortality and morbidity associated with RDS is a major advantage. By effectively addressing the underlying cause of RDS—surfactant deficiency—CUROSURF helps to minimize the long-term consequences of this condition, potentially improving the infants’ overall health and development. The reduction in long-term complications is a substantial clinical benefit.
Finally, the extensive clinical experience with CUROSURF provides a strong foundation for its continued use. Years of successful application in neonatal intensive care units worldwide have further established its safety and efficacy profile. This broad clinical experience ensures confidence in its use and effectiveness in managing this life-threatening condition.
Advantages of CUROSURF
CUROSURF presents several key advantages in the management of neonatal respiratory distress syndrome (RDS). Its natural composition, derived from porcine lungs, closely mimics the structure and function of endogenous surfactant, leading to a more physiological interaction with the infant’s delicate lung tissue. This natural origin minimizes the risk of adverse reactions often associated with synthetic alternatives.
The ready-to-use format of CUROSURF significantly streamlines the administration process. This eliminates the need for reconstitution, reducing preparation time and the potential for errors. The simplified administration is particularly beneficial in time-sensitive situations where rapid intervention is crucial for optimal patient outcomes. This ease of use is a significant advantage in busy neonatal intensive care units.
Extensive clinical trials have consistently demonstrated CUROSURF’s superior efficacy in improving oxygenation and reducing the need for mechanical ventilation. These improvements directly translate to better respiratory function and reduced risk of long-term respiratory complications for the infants. The positive impact on clinical outcomes is well-documented.
Importantly, CUROSURF has shown a marked reduction in mortality and morbidity associated with RDS. By effectively addressing the underlying surfactant deficiency, CUROSURF contributes to improved survival rates and reduces the severity of long-term health issues often associated with RDS. The impact on long-term health is a substantial clinical benefit.
The extensive clinical experience supporting CUROSURF’s use further strengthens its position as a preferred treatment option. Years of successful application in neonatal intensive care units across the globe have solidified its safety profile and reinforced its efficacy in managing this critical condition. This wealth of clinical data contributes to the overall confidence in its use.
Cons
Potential Drawbacks of CUROSURF
While CUROSURF offers substantial benefits in treating neonatal respiratory distress syndrome (RDS), it’s crucial to acknowledge potential drawbacks. Although rare, transient bradycardia (slow heart rate) and hypotension (low blood pressure) can occur following administration. These typically resolve spontaneously but necessitate close monitoring of vital signs during and after treatment.
Airway obstruction, potentially caused by the surfactant itself or by improper administration techniques, represents another potential risk. Careful administration, utilizing established clinical protocols and employing experienced healthcare professionals, is essential to minimize this risk. Thorough training and adherence to protocols are critical for safe administration.
Although CUROSURF is derived from natural sources, the potential for allergic reactions, though rare, cannot be entirely ruled out. A thorough assessment of the infant’s medical history, including any known allergies, is important before administration. Careful monitoring for any signs of allergic reactions is also necessary.
The cost of CUROSURF can be a significant factor for some healthcare systems. This financial consideration requires careful evaluation in resource allocation decisions, particularly in settings with limited healthcare budgets. Cost-effectiveness analyses may be necessary to balance the benefits of the treatment against its cost.
Finally, it’s important to note that CUROSURF, like any medication, does not guarantee a complete absence of adverse events. Individual responses can vary, and while the overall safety profile is generally favorable, the potential for unforeseen complications necessitates vigilant patient monitoring and prompt intervention if necessary. Careful observation and prompt action are key to managing potential complications.
-
 Georgia Austin [Author]
Georgia Austin [Author]Georgia Austin is a seasoned SEO content writer, editor, and content marketing strategist with over 7 years of experience crafting compelling copy for leading brands in the healthcare and pharmaceutic...
View all posts
-
 Jonathan Brown [Editor]
Jonathan Brown [Editor]Jonathan Brown is a seasoned professional editor, researcher, and educator with over 12 years of experience helping authors find their voice and polish their writing. As a content editor for RxPulsar....
View all posts
-
 Elizabeth Dennis, MD [Medical reviewer]
Elizabeth Dennis, MD [Medical reviewer]Dr. Elizabeth Dennis is a highly skilled Orthopedic Surgeon and consultant for RxPulsar.com, a licensed online pharmacy. She specializes in the management and surgical treatment of knee, shoulder, and...
View all posts















Reviews
There are no reviews yet.