Table of Contents
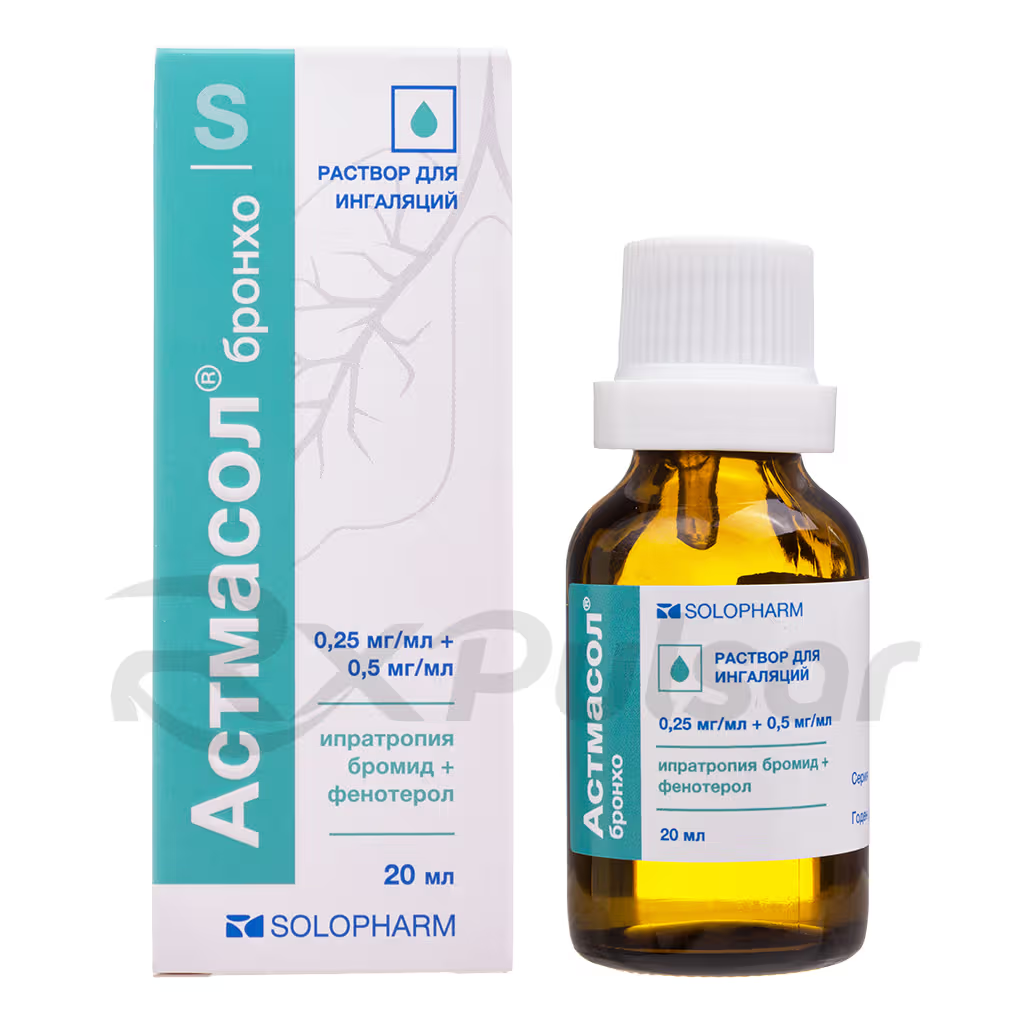
ASTMASOL™ BRONCHO 0.25mg+0.5mg Solution Buy Online
Astmasol Broncho Inhalation Solution: A Comprehensive Overview
Experiencing respiratory distress? Astmasol Broncho Inhalation Solution offers a potential solution for managing various respiratory conditions. This comprehensive overview explores its properties, uses, and considerations.
Astmasol Broncho is a combination bronchodilator, delivered as an inhalation solution. It effectively addresses bronchospasm, a major symptom in conditions like asthma and chronic obstructive pulmonary disease (COPD).
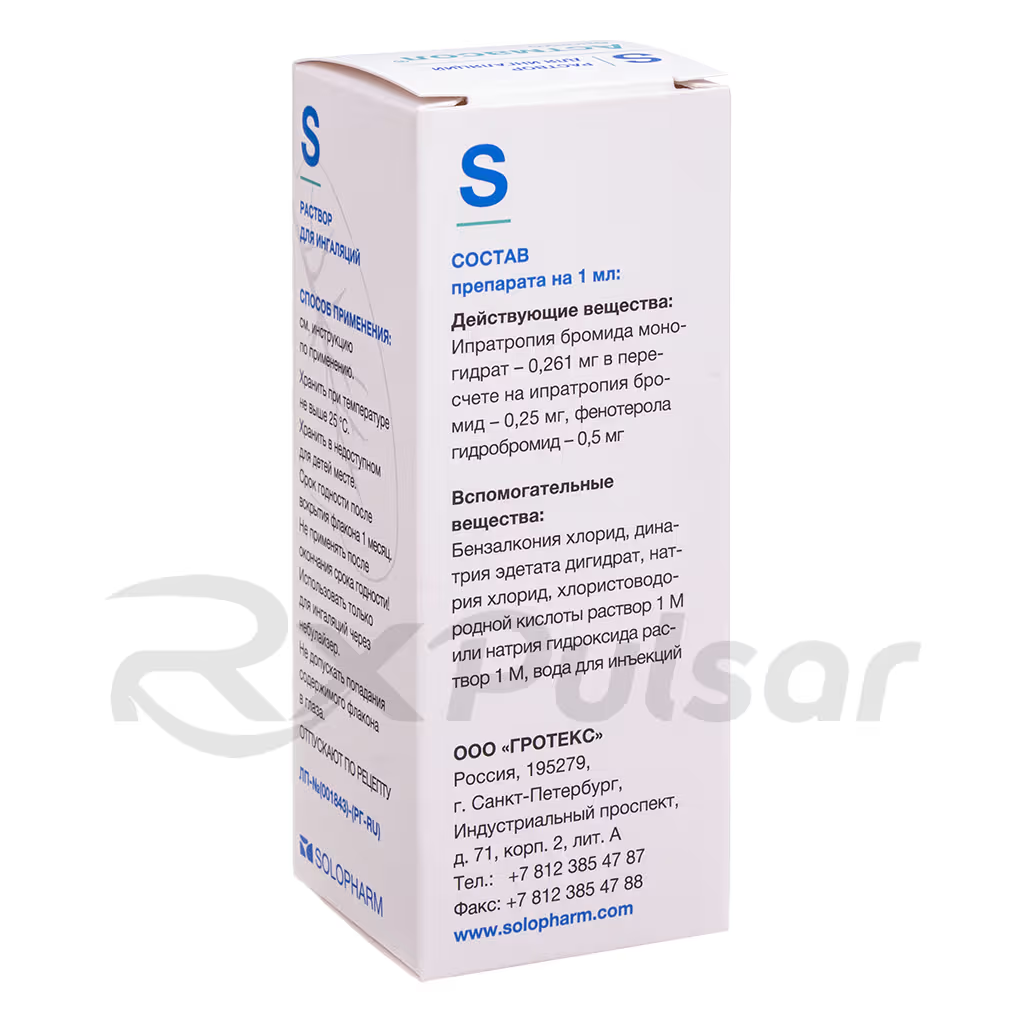
The solution comes in a convenient 20ml vial with a jet dispenser, ensuring accurate dosage. It’s crucial to understand that the solution should be diluted with 0.9% sodium chloride solution before each use, and any leftover diluted solution must be discarded.
What is Astmasol Broncho?
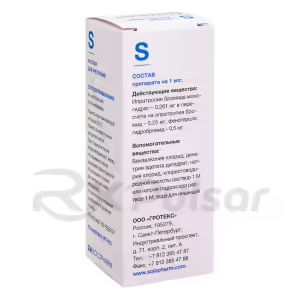
Astmasol Broncho Inhalation Solution is a combination medication designed to provide bronchodilation, the widening of the airways in the lungs. This action alleviates the symptoms associated with bronchospasm, a narrowing of the airways that makes breathing difficult. The formulation combines two active ingredients: ipratropium bromide, an anticholinergic agent, and fenoterol, a beta2-adrenergic agonist. These two components work synergistically to achieve a broader and more effective bronchodilating effect than either could achieve alone.
Ipratropium bromide, a muscarinic receptor antagonist, blocks the action of acetylcholine, a neurotransmitter that causes bronchoconstriction. This action helps relax the muscles surrounding the airways. Fenoterol, a selective beta2-adrenergic agonist, stimulates beta2-receptors in the lungs, resulting in smooth muscle relaxation and bronchodilation. The combined action of these two active components provides a longer-lasting and more potent bronchodilating effect compared to using either component individually. The solution is administered via inhalation using a nebulizer, ensuring that the medication directly reaches the affected airways for optimal efficacy.
Importantly, Astmasol Broncho is intended for the symptomatic treatment of chronic obstructive pulmonary diseases (COPD) with reversible airway obstruction, including asthma and chronic bronchitis. The precise mechanism of action involves the targeted relaxation of bronchial smooth muscle, thus improving airflow and reducing respiratory symptoms. This dual mechanism of action allows for both rapid relief and sustained bronchodilation, making it a versatile treatment option for various respiratory conditions. The provided 20ml vial is designed for convenient and controlled administration using a suitable nebulizer.
Mechanism of Action
Astmasol Broncho’s efficacy stems from the synergistic action of its two key components: ipratropium bromide and fenoterol. Ipratropium bromide, a potent anticholinergic agent, works by competitively blocking muscarinic receptors in the airways. This inhibition prevents the binding of acetylcholine, a neurotransmitter that triggers bronchoconstriction, leading to the relaxation of airway smooth muscles and subsequent bronchodilation. The effect is primarily localized to the respiratory tract, minimizing systemic side effects.
Fenoterol, a selective beta2-adrenergic agonist, complements ipratropium bromide’s action. It stimulates beta2-adrenergic receptors on airway smooth muscle cells, triggering a cascade of intracellular events that ultimately lead to muscle relaxation and bronchodilation. This mechanism complements ipratropium bromide’s action, resulting in a more comprehensive and sustained bronchodilation. The combined effect provides both rapid relief from bronchospasm and prolonged protection against airway narrowing. This dual mechanism of action allows for a more complete response to bronchoconstriction, addressing both cholinergic and non-cholinergic components.
The combined action of these two pharmacologically distinct agents results in a significant improvement in lung function. This is evidenced by increases in forced expiratory volume in 1 second (FEV1) and peak expiratory flow (PEF). The bronchodilatory effects of fenoterol typically last for 3-5 hours, while those of ipratropium bromide can persist for up to 6 hours, providing extended relief from respiratory symptoms. Importantly, the bronchodilation achieved through Astmasol Broncho is predominantly localized to the respiratory system, minimizing the risk of significant systemic effects.
Therapeutic Effects and Uses
Astmasol Broncho Inhalation Solution is primarily indicated for the relief of bronchospasm in patients with reversible airway obstruction. Its dual mechanism of action, combining anticholinergic and beta2-adrenergic effects, allows for effective management of both acute and chronic respiratory symptoms. The rapid onset of action makes it particularly useful in managing acute exacerbations of asthma or COPD, providing quick relief from breathlessness and wheezing. This makes it suitable for both short-term relief during acute episodes and long-term management of chronic conditions under medical supervision.
The sustained bronchodilation provided by Astmasol Broncho offers significant therapeutic benefits. Patients experience improved airflow, reduced breathlessness, and an enhanced quality of life. The prolonged effects of ipratropium bromide and fenoterol ensure that relief is sustained for several hours, minimizing the frequency of medication administration. This extended relief allows for improved sleep quality and better participation in daily activities for those with chronic respiratory issues. Regular use, as prescribed by a healthcare professional, can help prevent future exacerbations and maintain optimal lung function.
Astmasol Broncho is particularly beneficial for patients who experience inadequate relief from beta2-agonists alone or who cannot tolerate or effectively use aerosol inhalers. Its use in these situations allows for better management of respiratory symptoms and improved overall respiratory health. The solution’s delivery via nebulizer ensures efficient drug delivery to the lungs, enhancing its therapeutic efficacy. However, it’s essential to remember that Astmasol Broncho should only be used under the guidance of a physician, who will determine the appropriate dosage and treatment regimen based on the individual patient’s needs and medical history. This ensures the safe and effective use of this powerful bronchodilator.
Dosage and Administration
Astmasol Broncho is administered via inhalation using a suitable nebulizer. The solution must be diluted with a 0.9% sodium chloride solution before each use; never use distilled water. The recommended dilution is to a final volume of 3-4ml. Dosage is individualized based on the severity of the symptoms and the patient’s age and weight, always under the guidance of a healthcare professional.
For adults and adolescents over 12 years old, the initial dose may range from 1ml (20 drops) to 2.5ml (50 drops), potentially increasing to 4ml (80 drops) in severe cases. Children aged 6 to 12 years may receive a lower dose, typically starting at 0.5ml (10 drops) and increasing to a maximum of 2ml (40 drops) as needed. For children under 6 years old (weighing less than 22 kg), dosage should be determined by a physician and may involve 0.1ml (2 drops) per kilogram of body weight, not exceeding 0.5ml (10 drops). This lower dosage for younger children reflects the limited data available on their use of this medication.
Crucially, the diluted solution should be used immediately after preparation; any remaining solution must be discarded. The entire diluted dose should be administered via nebulization. It is critical to emphasize that this medication is for inhalation only and should never be taken orally. Dosage adjustments must be made based on the patient’s response to treatment and the severity of their symptoms. Close monitoring by a healthcare professional is essential to ensure both safety and efficacy, particularly in children and older adults.
Pros
Astmasol Broncho offers several key advantages in managing respiratory conditions. Its dual mechanism of action, combining ipratropium bromide and fenoterol, provides a broader and more effective bronchodilating effect than either agent alone. This translates to significant improvements in lung function, as measured by increased FEV1 and PEF, leading to noticeable relief from breathlessness and wheezing. The rapid onset of action is particularly beneficial during acute exacerbations, offering swift relief from severe respiratory distress.
The sustained bronchodilation provided by Astmasol Broncho is another significant advantage. The longer duration of action, lasting several hours, reduces the need for frequent dosing compared to other short-acting bronchodilators. This translates to improved convenience and adherence to the treatment regimen, leading to better overall respiratory symptom control. The prolonged relief allows for improved sleep quality and increased participation in daily activities, improving the overall quality of life for patients with chronic respiratory conditions.
Furthermore, Astmasol Broncho’s delivery via nebulization ensures efficient drug delivery to the lungs. This targeted delivery method optimizes the therapeutic effect while minimizing the potential for systemic side effects. Its suitability for patients who cannot tolerate or effectively use metered-dose inhalers provides an alternative route of administration, expanding the range of patients who can benefit from its bronchodilating effects. The combination of rapid onset, sustained relief, and efficient delivery makes Astmasol Broncho a valuable therapeutic option for a wide range of patients experiencing bronchospasm.
Cons
While Astmasol Broncho offers significant benefits, potential drawbacks should be considered. Like other bronchodilators, it carries the risk of paradoxical bronchospasm, a potentially life-threatening condition where airway narrowing worsens after medication use. This necessitates careful monitoring, especially in patients with a history of this reaction. The likelihood of this adverse event is generally low but warrants awareness and prompt medical attention if it occurs. It’s crucial that patients report any worsening of respiratory symptoms immediately to their healthcare provider.
Furthermore, Astmasol Broncho, due to its constituent components, can cause various side effects, although these are typically mild and transient. These can include tremor, nervousness, headache, and dry mouth. The anticholinergic properties of ipratropium bromide may also lead to urinary retention, especially in patients with pre-existing urinary tract conditions. Patients with a history of glaucoma, cardiovascular disease, hyperthyroidism, or prostate enlargement should use Astmasol Broncho with caution and under close medical supervision, as these conditions may be exacerbated by the medication’s effects.
Finally, the need for nebulization equipment represents a practical consideration. Patients require access to a nebulizer and the necessary supplies for proper administration. This can be a barrier for some individuals, particularly those without access to appropriate healthcare resources. The necessity of diluting the solution before each use adds an extra step to the administration process. While generally well-tolerated, potential side effects and logistical considerations should be carefully weighed against the therapeutic benefits when deciding on the appropriate treatment plan for individual patients.
Pharmacokinetics
Understanding the pharmacokinetic profile of Astmasol Broncho is crucial for optimizing its therapeutic use. Following inhalation, only a fraction of the administered dose (10-39%) reaches the lungs; the remainder deposits in the mouth, throat, or is swallowed. The portion deposited in the lungs is rapidly absorbed into the systemic circulation within minutes. Swallowed portions undergo significant first-pass metabolism, resulting in low oral bioavailability (around 1.5%). This means that the primary route of therapeutic effect is through direct pulmonary deposition and absorption.
Ipratropium bromide, being a quaternary ammonium compound, exhibits minimal plasma protein binding (less than 20%). It undergoes limited metabolism, primarily through hepatic oxidation, with a terminal half-life of approximately 1.6 hours. Excretion occurs mainly via the kidneys (approximately 60% of the intravenously administered dose). Fenoterol, on the other hand, demonstrates a higher degree of plasma protein binding (around 40%). It’s metabolized extensively, primarily forming sulfate conjugates, and is excreted via both the renal and biliary routes. Its terminal half-life is approximately 3 hours.
The pharmacokinetic profiles of ipratropium bromide and fenoterol in Astmasol Broncho are generally consistent with their individual profiles. However, it’s important to note that the observed bronchodilation is not directly proportional to the plasma concentrations of either component. This highlights the predominantly local action of the drug within the respiratory tract, emphasizing the importance of proper inhalation technique for optimal therapeutic efficacy. The combination of these two drugs results in sustained bronchodilation that is greater than the sum of their individual effects.
Precautions and Contraindications
Astmasol Broncho, while effective, necessitates careful consideration of potential risks and contraindications. It is contraindicated in patients with known hypersensitivity to fenoterol, atropine-like drugs, or any other component of the formulation. Individuals with hypertrophic obstructive cardiomyopathy should also avoid this medication due to potential cardiovascular effects. Use in patients with other significant underlying health issues requires careful assessment by a healthcare professional to balance the benefits against potential risks.
Caution is advised in patients with certain pre-existing conditions. These include narrow-angle glaucoma, as the anticholinergic effects of ipratropium bromide may exacerbate this condition. Similarly, patients with arterial hypertension, diabetes mellitus, recent myocardial infarction (within the last three months), significant cardiovascular disease (including chronic heart failure, coronary artery disease, aortic stenosis), severe cerebrovascular or peripheral artery disease, hyperthyroidism, pheochromocytoma, prostatic hyperplasia, bladder neck obstruction, cystic fibrosis, or those who are pregnant (second and third trimesters) or breastfeeding should use Astmasol Broncho only under strict medical supervision. Close monitoring of vital signs and potential side effects is necessary in these populations.
Furthermore, children under 6 years of age should only receive Astmasol Broncho under strict medical supervision due to limited data on its use in this age group. Administration should always be performed by or under the direct supervision of an adult. The potential for paradoxical bronchospasm is another key consideration; patients should be closely monitored for any worsening of respiratory symptoms following administration. Any unusual or concerning side effects should be promptly reported to a physician. The decision to use Astmasol Broncho should always be based on a thorough risk-benefit assessment tailored to the individual patient’s clinical circumstances.
Conclusion
Astmasol Broncho Inhalation Solution presents a valuable therapeutic option for managing bronchospasm in individuals with reversible airway obstruction. Its unique combination of ipratropium bromide and fenoterol offers a potent and sustained bronchodilating effect, providing rapid relief from acute symptoms and longer-term control of chronic conditions. The dual mechanism of action targets both cholinergic and non-cholinergic pathways, leading to a more comprehensive and effective treatment approach compared to single-agent therapies. However, the potential for paradoxical bronchospasm and other adverse effects necessitates careful consideration of its use and close monitoring by healthcare professionals.
The advantages of Astmasol Broncho, including its rapid onset of action and prolonged duration of effect, contribute significantly to improved patient outcomes. The nebulizer delivery system allows for efficient drug delivery directly to the lungs, optimizing therapeutic efficacy while minimizing systemic side effects. However, the need for nebulization equipment, the requirement for dilution before each use, and the potential for adverse reactions highlight the importance of using this medication only under the guidance of a physician. Individualized dosage adjustments and careful patient monitoring are essential to maximize therapeutic benefits while minimizing the risk of adverse events.
In summary, Astmasol Broncho offers a powerful tool for managing bronchospasm, but its use requires careful consideration of potential risks and benefits. Appropriate patient selection, meticulous administration, and close medical supervision are crucial for ensuring safe and effective treatment. The decision to use Astmasol Broncho should be made in consultation with a healthcare professional who can assess the individual patient’s needs and medical history to determine the suitability of this medication and to establish a safe and effective treatment plan. This comprehensive approach will ensure optimal patient care and minimize the potential for complications.
-
 Georgia Austin [Author]
Georgia Austin [Author]Georgia Austin is a seasoned SEO content writer, editor, and content marketing strategist with over 7 years of experience crafting compelling copy for leading brands in the healthcare and pharmaceutic...
View all posts
-
 Jonathan Brown [Editor]
Jonathan Brown [Editor]Jonathan Brown is a seasoned professional editor, researcher, and educator with over 12 years of experience helping authors find their voice and polish their writing. As a content editor for RxPulsar....
View all posts
-
 David J Bronster, MD [Medical reviewer]
David J Bronster, MD [Medical reviewer]Dr. David J. Bronster, MD, is a distinguished Professor of Neurology and Neurological Consultant to the Recanati/Miller Transplantation Institute. With an impressive 36-year career in consultative wor...
View all posts
















Reviews
There are no reviews yet.