Table of Contents
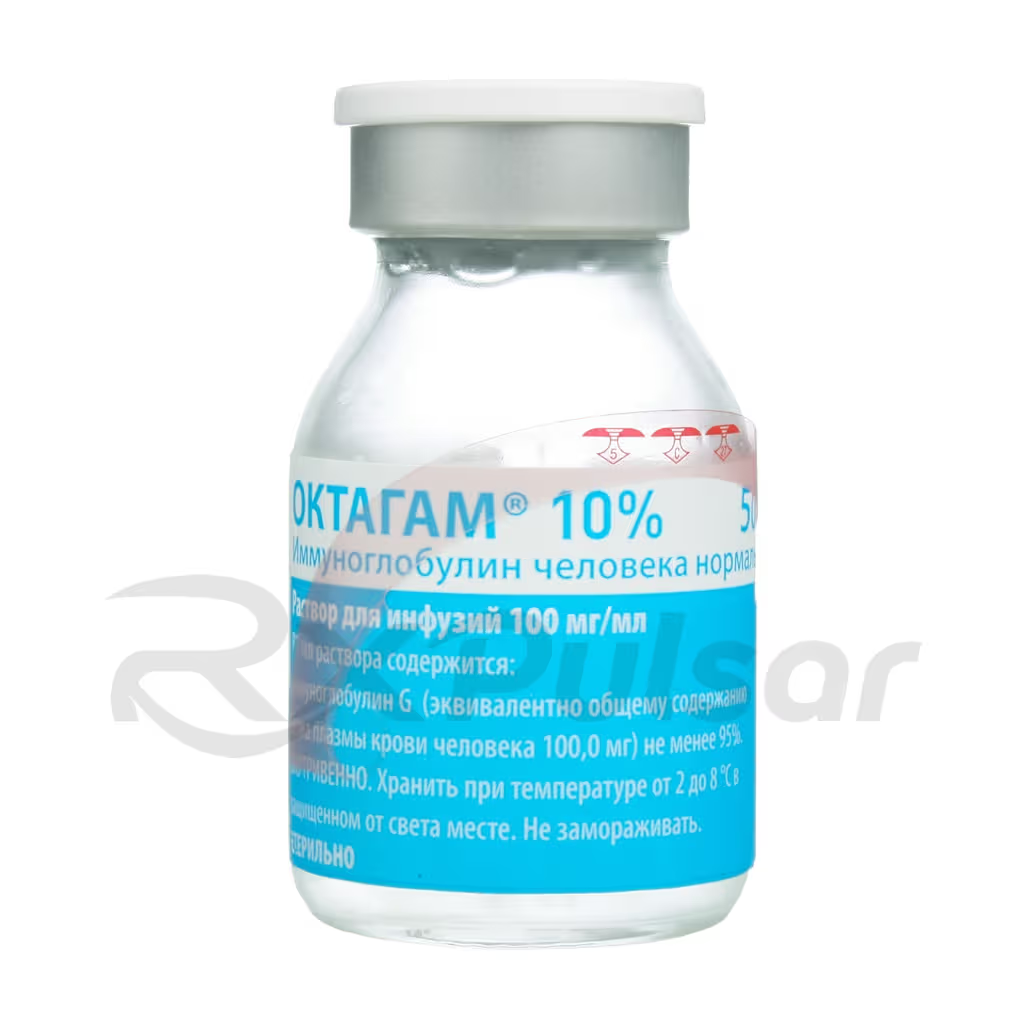
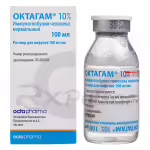
IMMUNOGLOBULIN 10% 100mg/ml Solution 50ml Buy Online
Normal Human Immunoglobulin 10% Infusion Solution
Normal human immunoglobulin (Ig) is a vital component of the immune system, providing crucial defense against infections. This 10% infusion solution offers a concentrated form of this essential protein, delivering targeted support where needed.
This concentrated solution is designed for intravenous administration, offering a convenient and effective method for delivering immunoglobulin therapy. The precise concentration allows for accurate dosage adjustments based on individual patient needs and clinical situations. Its effectiveness lies in its ability to bolster the body’s natural immune response.
The 50ml vial provides a manageable unit for administration, suitable for various treatment protocols. The 100mg/ml concentration ensures efficient delivery of a high dose of immunoglobulin within a relatively small volume, minimizing the infusion time and enhancing patient comfort. Proper handling and administration are crucial for optimal results.
Understanding Normal Human Immunoglobulin
Normal Human Immunoglobulin (Ig), a crucial component of the immune system, is a complex mixture of antibodies derived from the pooled plasma of many healthy donors. These antibodies, primarily Immunoglobulin G (IgG), play a vital role in protecting the body against a wide array of infections. Its effectiveness stems from its ability to neutralize pathogens and enhance the body’s natural defense mechanisms.
The immunoglobulin preparation undergoes rigorous processing to ensure safety and efficacy, removing potentially harmful substances while retaining the essential antibody components. This purification process is crucial for minimizing the risk of adverse reactions and maximizing therapeutic benefits. The resulting solution, as in the case of a 10% infusion solution, offers a highly concentrated form of these vital antibodies.
Different immunoglobulin preparations, such as those used for intravenous administration, may vary slightly in their composition and concentration. However, the core function remains consistentâto provide passive immunity, supplementing or replacing deficient antibody levels in individuals with impaired immune systems. Understanding this fundamental role is key to appreciating the therapeutic applications of normal human immunoglobulin.
Administration of normal human immunoglobulin is a well-established procedure, used to treat a range of immune deficiencies and related conditions. The method of delivery, whether intravenous or subcutaneous, is carefully chosen based on individual patient needs and the severity of the condition. Careful monitoring during and after administration is crucial for ensuring patient safety and effectiveness of treatment.
The specific properties of the immunoglobulin, including its concentration and the method of preparation, are carefully controlled to guarantee consistent quality and efficacy. These quality controls ensure that each batch meets stringent standards, maintaining the high level of safety and efficacy expected in these life-sustaining therapies. Continuous research and development further enhance the understanding and improvement of these vital preparations.
Therapeutic Applications
Normal human immunoglobulin 10% infusion solutions find broad application in treating various immune deficiencies and related conditions. These solutions are particularly useful in situations where the body’s own immune system is compromised or unable to produce sufficient antibodies to fight off infections effectively. This targeted therapy helps restore immune balance and protect against recurrent infections.
One significant application lies in managing primary immunodeficiency disorders. These disorders, often genetic in origin, result in a severely weakened immune system leaving individuals vulnerable to frequent and severe infections. Intravenous immunoglobulin (IVIG) infusions, such as the 10% solution, provide a crucial source of antibodies, bolstering the body’s defenses and mitigating infection risk.
Beyond primary immunodeficiencies, IVIG is also valuable in treating secondary immunodeficiencies. These deficiencies can arise from various factors, including certain cancers, HIV infection, and certain medications. By supplementing antibody levels, IVIG can help reduce the frequency and severity of infections in individuals with these conditions, significantly improving their quality of life.
Furthermore, IVIG therapy proves beneficial in managing certain autoimmune disorders. In these conditions, the immune system mistakenly attacks the body’s own tissues. While not a cure, IVIG can modulate the immune response, reducing inflammation and alleviating symptoms. This approach is particularly relevant in conditions where other treatments have limited effectiveness.
Specific conditions where IVIG may be employed include, but are not limited to, idiopathic thrombocytopenic purpura (ITP), a bleeding disorder characterized by low platelet counts, and certain neurological disorders associated with autoimmunity. The precise application and dosage of IVIG are determined on a case-by-case basis by medical professionals, based on the individual patient’s needs and the specific condition being treated.
The versatility of IVIG treatment makes it a valuable therapeutic option across a spectrum of immune-related conditions. Its ability to directly address antibody deficiencies and modulate immune responses offers significant therapeutic benefits for patients with compromised immune systems or autoimmune disorders. Further research continues to expand our understanding of its applications and potential benefits.
Administration and Dosage
The administration of normal human immunoglobulin 10% infusion solution, like other intravenous immunoglobulin (IVIG) products, requires careful attention to detail to ensure patient safety and efficacy. This involves meticulous preparation, precise infusion rates, and vigilant monitoring for potential adverse reactions. The process should always be carried out under the supervision of qualified healthcare professionals.
Prior to administration, the solution should be inspected visually for any particulate matter or discoloration. If any abnormalities are detected, the solution should not be used. The solution should be allowed to reach room temperature before administration to enhance patient comfort and minimize discomfort during the infusion. This warming process should be done carefully, avoiding excessive heat.
Infusion rate is a critical factor influencing both safety and efficacy. Generally, the initial infusion rate is relatively slow to assess for any immediate adverse reactions. If well-tolerated, the rate can be gradually increased. However, exceeding recommended rates can increase the risk of side effects, including headache, fever, and chills. Healthcare professionals closely monitor patients during the infusion.
Dosage regimens vary significantly depending on the specific indication, the patient’s weight, and the severity of their condition. For instance, treatment for primary immunodeficiency often involves regular infusions to maintain adequate antibody levels. The frequency and amount of immunoglobulin administered are determined by the treating physician based on individual patient needs and response to treatment. Regular blood tests help monitor antibody levels and guide dosage adjustments.
In certain conditions, such as idiopathic thrombocytopenic purpura (ITP), a single high-dose infusion may be sufficient, while other conditions may require a series of infusions. Healthcare providers carefully consider the individual circumstances, weighing potential benefits against potential risks to determine the most appropriate treatment plan. Close monitoring ensures the optimal balance between effective therapy and minimizing potential side effects.
Accurate record-keeping is essential throughout the treatment process. This includes documenting the date, time, dose administered, infusion rate, and any observed reactions. This detailed record assists in tracking treatment progress and identifying potential patterns or trends relevant to individual patient response. The information compiled is crucial for ongoing management and optimization of therapy.
Potential Side Effects
While generally well-tolerated, intravenous immunoglobulin (IVIG) therapy, including the 10% infusion solution, can cause a range of side effects. These reactions vary in severity, from mild and transient to more serious, though serious adverse events are relatively uncommon. Careful monitoring during and after infusion is crucial to promptly identify and manage any potential issues.
Common side effects often include headache, nausea, fever, chills, and muscle aches. These are typically mild and self-limiting, resolving spontaneously within a short period. However, patients should report any discomfort to healthcare providers, who may adjust the infusion rate or provide symptomatic relief. Simple measures like slowing the infusion rate can often alleviate these symptoms.
Less frequent but more serious side effects can include allergic reactions, ranging from mild rash to life-threatening anaphylaxis. Patients with a history of allergies or hypersensitivity reactions should be carefully monitored during the infusion. Prompt recognition and treatment of allergic reactions are paramount, requiring immediate intervention by healthcare professionals.
Other potential side effects, though rare, include thromboembolic events (blood clots), primarily in patients with pre-existing risk factors. These events can be serious and require prompt medical attention. Patients with a history of clotting disorders or those at increased risk should be carefully assessed before initiating IVIG therapy. Regular monitoring for any signs or symptoms of clotting is important.
Renal dysfunction is another potential complication, particularly in patients with pre-existing kidney problems or receiving high doses of IVIG. Close monitoring of kidney function through blood tests is advisable, especially in vulnerable populations. Adjusting the dosage or frequency of infusions may be necessary to mitigate this risk. Healthcare providers carefully assess this risk before initiating treatment.
Aseptic meningitis, a rare but serious complication characterized by inflammation of the brain and spinal cord membranes, has been associated with IVIG administration. This condition typically presents with headache, fever, and neck stiffness. Prompt diagnosis and treatment are crucial to minimize potential long-term neurological consequences. Healthcare providers are trained to recognize this complication and initiate appropriate medical interventions.
Precautions and Contraindications
While normal human immunoglobulin 10% infusion solution offers significant therapeutic benefits, certain precautions and contraindications must be considered to ensure patient safety and optimize treatment outcomes. Careful assessment of the patient’s medical history and current health status is crucial before initiating therapy. This includes a thorough review of potential allergies, existing medical conditions, and any concurrent medications.
Patients with a known history of hypersensitivity to human immunoglobulins or any components of the formulation should avoid using this product. Even patients without a documented allergy should be closely monitored during the initial infusion for any signs of hypersensitivity. Healthcare providers are trained to recognize and manage such reactions, including allergic reactions and anaphylaxis.
Individuals with a history of severe heart or kidney disease may be at increased risk of complications from IVIG administration. Careful evaluation of the benefit-risk ratio is necessary in such cases. Close monitoring of vital signs and organ function is essential during and after the infusion in these patients. Treatment plans should consider individual circumstances and risk profiles.
Pregnancy and breastfeeding should be carefully considered before administering IVIG. While generally considered safe, the potential risks and benefits should be carefully weighed. The decision to use IVIG during pregnancy or breastfeeding should be made in consultation with healthcare professionals, considering the specific clinical circumstances. There is limited data on long-term effects in infants.
Patients receiving concurrent medications, particularly those affecting the immune system or kidney function, may require dosage adjustments or close monitoring. Potential drug interactions should be assessed to avoid adverse effects. Healthcare providers should review the patient’s complete medication list to identify potential conflicts and devise a safe treatment plan.
Finally, individual patient factors such as age, weight, and underlying health conditions will influence the dosage and frequency of IVIG infusions. Healthcare professionals tailor treatment plans to individual patient needs, ensuring optimal therapeutic outcomes while minimizing potential risks. Regular monitoring of patient response and careful adjustment of treatment parameters are key to successful therapy.
Pros
Normal human immunoglobulin 10% infusion solution offers several key advantages in the treatment of various immune-related conditions. Its efficacy in restoring antibody levels and modulating immune responses makes it a valuable therapeutic option for patients with compromised immune systems or autoimmune disorders. The concentrated formulation ensures efficient delivery of a high dose of immunoglobulin within a relatively small volume.
One major advantage is its ability to provide immediate immune support. Unlike other therapies that may take time to build up immune response, IVIG provides a rapid boost to antibody levels, offering immediate protection against infections. This is particularly crucial in patients with severe immunodeficiencies or those facing acute infections. The rapid action offers a significant advantage in urgent situations.
The versatility of IVIG treatment is another significant benefit. It’s effective across a range of conditions, from primary immunodeficiencies to autoimmune disorders, offering a single therapeutic approach for diverse clinical scenarios. This broad applicability simplifies treatment management and reduces the need for multiple therapies. This reduces the complexity of treatment regimes.
Furthermore, the well-established safety profile of IVIG, with decades of clinical use, provides confidence in its application. While side effects can occur, they are generally manageable and relatively uncommon, especially with proper administration and monitoring. The extensive clinical experience contributes to its widespread acceptance among healthcare professionals.
The convenience of intravenous administration contributes to its widespread use. The concentrated solution allows for efficient delivery of high doses of immunoglobulin, minimizing infusion time and enhancing patient comfort. This contrasts with other delivery methods which may be more time consuming and less convenient for patients. The improved convenience benefits both patients and healthcare providers.
Finally, ongoing research and development continue to refine IVIG production methods and broaden our understanding of its therapeutic applications. This ensures that the quality, safety, and efficacy of IVIG preparations remain at the forefront of medical advancements, constantly improving the treatment experience and outcomes for patients. This ongoing commitment to improvement ensures its continued relevance in modern medicine.
Cons
Despite its numerous benefits, the use of normal human immunoglobulin 10% infusion solution is not without potential drawbacks. While serious adverse events are uncommon, the possibility of side effects necessitates careful patient selection, meticulous administration, and vigilant monitoring throughout the treatment process. Understanding these potential limitations is crucial for informed decision-making.
One significant limitation is the potential for adverse reactions. While many side effects are mild and self-limiting, such as headache or chills, more serious reactions, including allergic reactions and anaphylaxis, can occur. The risk of these serious reactions necessitates close monitoring during and after infusion, with appropriate medical intervention readily available. This requires a prepared medical team.
Another potential drawback is the cost of IVIG therapy. The high production costs and the often-substantial dosage requirements can make IVIG a relatively expensive treatment option. This cost factor can impact accessibility, particularly for patients with limited financial resources or those requiring long-term therapy. Cost-effectiveness analyses may be needed in certain situations.
The potential for transmission of infectious agents, although extremely rare due to rigorous screening and processing of donor plasma, remains a theoretical risk. While the probability is low, it underscores the importance of stringent manufacturing standards and careful donor selection. The rigorous testing and safety measures mitigate this risk.
Furthermore, IVIG therapy may not be suitable for all patients. Individuals with certain pre-existing conditions, such as severe heart or kidney disease, may be at increased risk of complications. Careful assessment of the benefit-risk ratio is crucial in such cases. Appropriate patient selection is essential for safe administration.
Finally, the need for intravenous administration can be a logistical challenge. This may require hospitalization or frequent visits to a healthcare facility, which can be inconvenient and burdensome for patients. This contrasts with some other therapies that can be self-administered at home. The need for medical supervision presents a logistical challenge.
-
 Georgia Austin [Author]
Georgia Austin [Author]Georgia Austin is a seasoned SEO content writer, editor, and content marketing strategist with over 7 years of experience crafting compelling copy for leading brands in the healthcare and pharmaceutic...
View all posts
-
 Jonathan Brown [Editor]
Jonathan Brown [Editor]Jonathan Brown is a seasoned professional editor, researcher, and educator with over 12 years of experience helping authors find their voice and polish their writing. As a content editor for RxPulsar....
View all posts
-
 Jessica Kerns, MD [Medical reviewer]
Jessica Kerns, MD [Medical reviewer]Dr. Jessica Kerns is a highly accomplished pediatrician and adolescent medicine specialist who serves as a clinical instructor in the Department of Pediatrics at the Icahn School of Medicine at Mount...
View all posts










Reviews
There are no reviews yet.